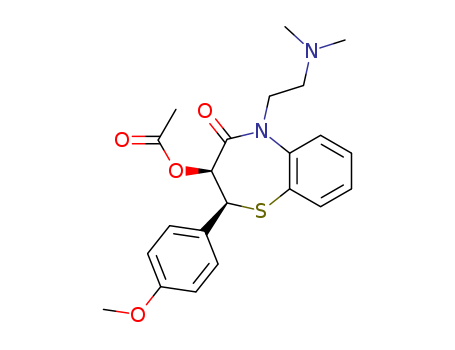
CasNo: 42399-41-7
Molecular Formula: C22H26N2O4S
|
Originator |
Herbesser,TANABE SEIYAKU,Japan,1974 |
|
Uses |
Diltiazem is a medication classified as a calcium channel blocker. Diltiazem is used for both stable and unstable angina pectoris. This includes cases occurring after myocardial infarctions (heart attacks). By reducing the workload on the heart, Diltiazem helps alleviate chest pain associated with angina. |
| Effects on Calcium Influx | Diltiazem reduces the influx of calcium ions across cell membranes in cardiac muscle and smooth muscle cells of blood vessels. This action contributes to its vasodilatory effects and helps prevent coronary artery spasms. |
| Blood Pressure Reduction | By dilating coronary and peripheral vessels and reducing the force and rate of the heartbeat, Diltiazem helps lower elevated arterial pressure and control tachycardia (rapid heart rate). |
|
Definition |
ChEBI: A 5-[2-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydro-1,5-benzothiazepin-3-yl acetate in which both stereocentres have S configuration. A calcium-channel blocker and vasodilator, it is used as the hydrochloride in the m nagement of angina pectoris and hypertension. |
|
Therapeutic Function |
Coronary vasodilator |
|
Synthesis |
Diltiazem, 5-[2-(diethylamino)ethyl]-cis-2,3-dihydro-3-hydroxy-2- (4-methoxy-phenyl)-1,5-benzothiazepin-4(5H)-one (19.3.10), is synthesized in the following manner. The condensation of 4-methoxybenzaldehyde with methylchoroacetate in the presence of sodium methoxide in Darzens reaction conditions gives methyl ester of 3- (4-methoxyphenyl)-glycidylic acid (19.3.5). Reacting it with 2-aminothiophenol with the opening of epoxide ring gives methyl ester of 2-hydroxy-3-(2'-aminophenylthio)-3- (4"- methoxyphenyl)propionic acid (19.3.6). Hydrolysis of the resulting compound with alkali leads to the formation of the corresponding acid (19.3.7) in the form of a racemic mixture, which when on interaction with (+)-α-phenylethylamine gives threo-(+)-2-hydroxy-3-(2'- aminophenylthio)-3-(4"-methoxyphenylpropionic acid (19.3.8). Boiling this in a mixture of acetic anhydride/dimethylformamide/pyridine system brings to cyclization to the thiazepine ring and simultaneously acylates the hydroxyl group, forming (+)-cis-2-(4-methoxyphenyl)- 3-acetoxy-2,3-dihydro-1,5-benzothiazepin-4-(5H)-one (19.3.9). Alkylation of the resulting product with 2,2-dimethylaminoethylchloride forms diltiazem (19.3.10). |
InChI:InChI=1/C22H26N2O4S/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3/h5-12,20-21H,13-14H2,1-4H3/t20-,21+/m1/s1
Life table analysis in patients with an EF of less than 0.40 confirmed more frequent late CHF in those taking diltiazem (p = 0.0017). In addition, the diltiazem-associated rise in the frequency of late CHF was progressively greater with increasingly severe decrements in baseline EF. This diltiazem effect was absent in patients with pulmonary congestion at baseline but an EF of 0.40 or more, suggesting a unique association between diltiazem-related late CHF and systolic LVD...
Diltiazem (DIL) is a calcium channel blo...
Diltiazem is an orally and intravenously active calcium channel blocking agent shown to be an effective and well- tolerated treatment for stable angina and angina due to coronary artery spasm. Its efficacy in these diseases has generally been similar to that of nifedipine or verapamil — alternative calcium channel blockers with which diltiazem has many electrophysiological, haemodynamic, and antiarrhythmic similarities.
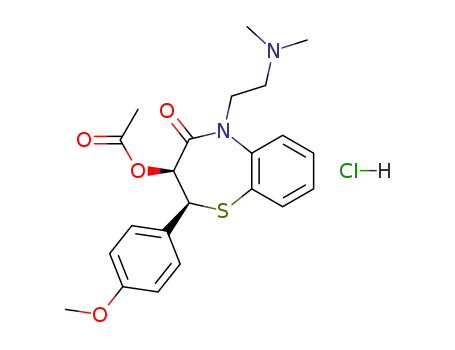
diltiazem hydrochloride

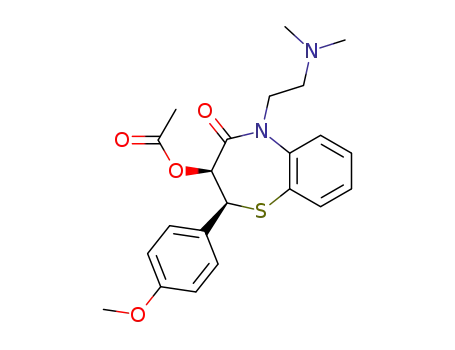
diltiazem
| Conditions | Yield |
|---|---|
|
With sodium carbonate; In water; for 0.333333h;
|
85% |
|
With sodium hydrogencarbonate; In water; ethyl acetate;
|
|
|
With sodium carbonate; In water; pH=7.5;
|
|
|
With sodium hydrogencarbonate; In water; for 0.25h;
|
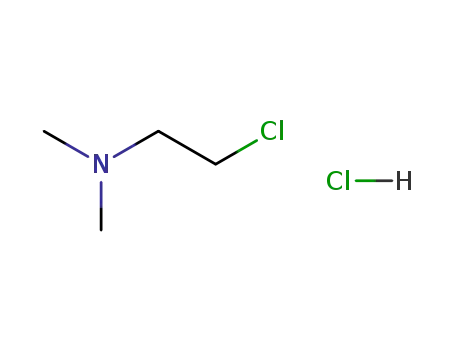
(2-chloroethyl)dimethylamine hydrochloride

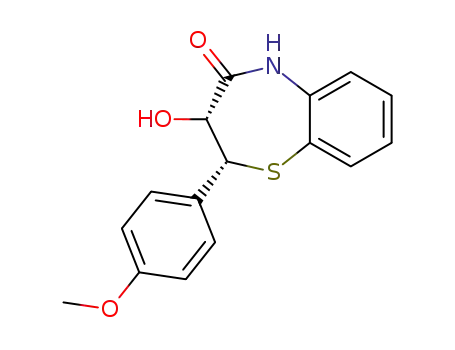
cis-(+)-2-(4'-methoxyphenyl)-3-hydroxy-2,3-dihydro-1,5-benzothiazepine-4(5H)-one

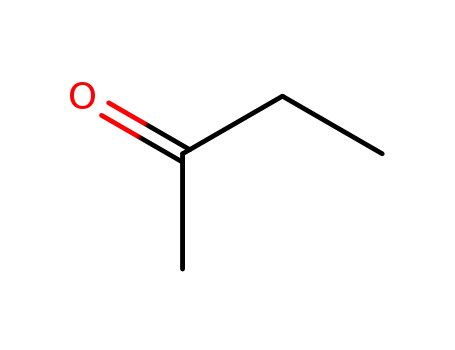
butanone

cis-(+)-3-hydroxy-5-[2-(dimethylamino)-ethyl]-2,3-dihydro-2-(4-methoxyphenyl-1,5-benzothiazepine-4(5H)-one


diltiazem
| Conditions | Yield |
|---|---|
|
With acetic anhydride; potassium carbonate; In water;
|
97.2% |
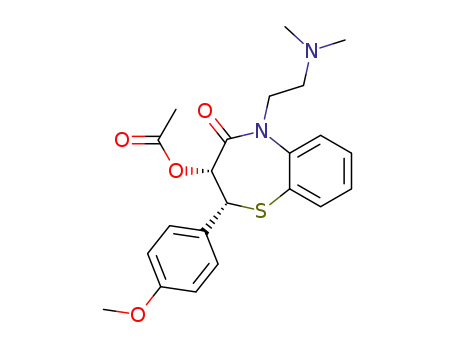
(+/-)-Diltiazem
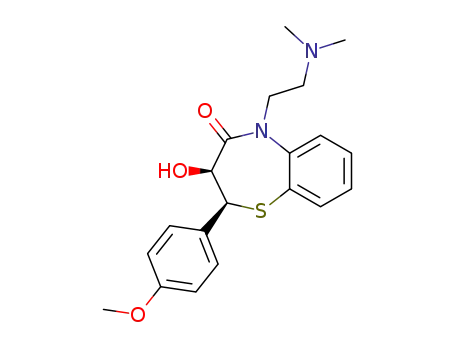
O-desacetyldiltiazem
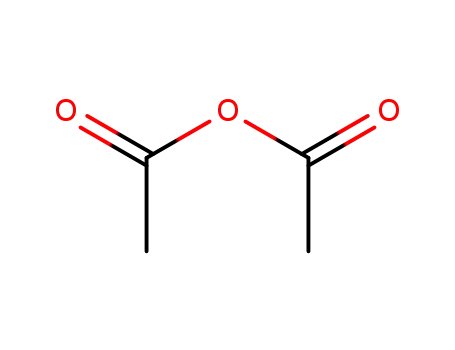
acetic anhydride
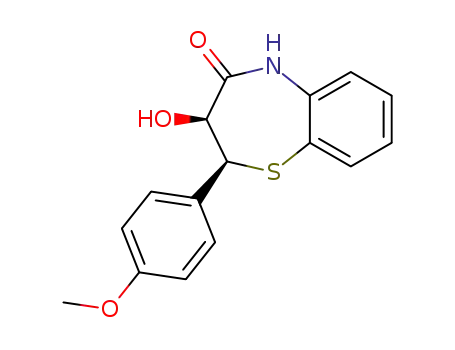
(2S,3S)-3-hydroxy-2-(4-methoxyphenyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one

O-desacetyldiltiazem
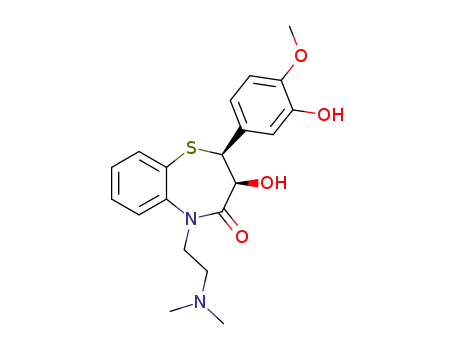
C20H24N2O4S
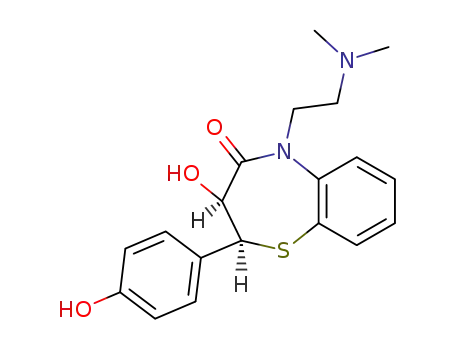
O-Desmethyldesacetyldiltiazem
N-desmethyldiltiazem