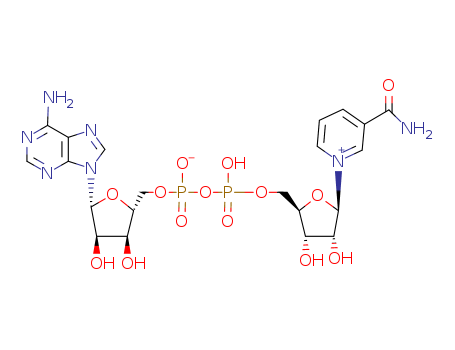
CasNo: 53-84-9
Molecular Formula: C21H27N7O14P2
Appearance: White powder
|
Description |
Beta-Diphosphopyridine Nucleotide (NAD+), also known as nicotinamide adenine dinucleotide, is a critical coenzyme and cofactor involved in various metabolic processes. |
| Chemical Composition |
NAD+ consists of two nucleotides joined through their phosphate groups. |
| Metabolic Significance |
Central to metabolism, playing a vital role in oxidation-reduction reactions. |
|
Definition |
ChEBI: β-Nicotinamide adenine dinucleotide (NAD) is the oxidized form of β-Nicotinamide Adenine Dinucleotide. It exists as an anion under normal physio-logic conditions. It is functionally related to a deamido-NAD zwitterion. It is a conjugate base of a NAD(+). It is found widely in nature and is involved in numerous enzymatic reactions in which it serves as an electron carrier by being alternately oxidized (NAD+) and reduced (NADH). (Dorland, 27th ed) |
| Therapeutic Applications |
NAD+ therapy is known to boost energy levels and improve reaction times. |
| NAD+ as a Signaling Molecule | Functions as a signaling molecule in addition to its role as a cofactor. Essential for the activity of enzymes, including poly(ADP)-ribose polymerases and cADP-ribose synthases. |
|
Biochem/physiol Actions |
β-Nicotinamide adenine dinucleotide (β-NAD) is an electron carrier and a cofactor, significantly involved in enzyme-catalyzed oxido-reduction processes and many genetic processes. NAD cycles between the oxidized (NAD+) and reduced (NADH) forms to maintain a redox balance necessary for continued cell growth. NAD is also involved in microbial catabolism. β-NAD acts as a substrate for various enzymes in several cellular processes. |
|
Purification Methods |
NAD is purified by paper chromatography or better on a Dowex-1 ion-exchange resin. The column is prepared by washing with 3M HCl until free of material absorbing at 260nm, then with water, 2M sodium formate until free of chloride ions and, finally, with water. NAD, as a 0.2% solution in water, is adjusted with NaOH to pH 8, and adsorbed onto the column, washed with water, and eluted with 0.1M formic acid. Fractions with strong absorption at 360nm are combined, acidified to pH 2.0 with 2M HCl, and cold acetone (ca 5L/g of NAD) is added slowly and with constant agitation. It is left overnight in the cold, then the precipitate is collected in a centrifuge, washed with pure acetone and dried under vacuum over CaCl2 and paraffin wax shavings [Kornberg Methods Enzymol 3 876 1957]. It has been purified by anion-exchange chromatography [Dalziel & Dickinson Biochemical Preparations 11 84 1966.] The purity is checked by reduction to NADH (with EtOH and yeast alcohol dehydrogenase) which has 340mn 6220 M-1cm-1. [Todd et al. J Chem Soc 3727, 3733 1957.] [pKa, Lamborg et al. J Biol Chem 231 685 1958.] The free acid crystallises from aqueous Me2CO with 3H2O and has m 140-142o. It is stable in cold neutral aqueous solutions in a desiccator (CaCl2) at 25o, but decomposes at strong acid and alkaline pH. Its purity is checked by reduction with yeast alcohol dehydrogenase and EtOH to NADH and noting the OD at 340nm. Pure NADH (see below) has 340 6.2 x 104 M-1cm-1, i.e. 0.1mole of NADH in 3mL and in a 1cm path length cell has an OD at 340nm of 0.207. [Beilstein 26 IV 3644.] |
InChI:InChI=1/C21H27N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h1-4,7-8,10-11,13-16,20-21,29-32H,5-6H2,(H5-,22,23,24,25,33,34,35,36,37)/p-1/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1
In addition, all sera added to media containing beta-diphosphopyridine nucleotide (DPN) were … Beta-diphosphopyridine nucleotide* (DPN) powder was dissolved in sufficient distilled …
Commercial materials were collagenase, bovine albumin, glycerokinase, pyruvatc kinase, lactic dehydrogcnase, beta-diphosphopyridine nucleotide, phosphoenol pyruvic acid, …
The peroxidase-NADH oscillator examined ...
Enzymatic regulation of pyricline nucleo...
The Lys80, Gly82 and Met101 residues of ...
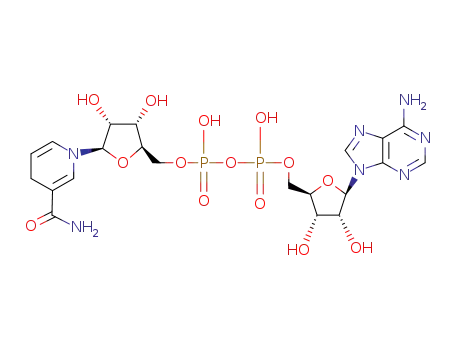
NADH

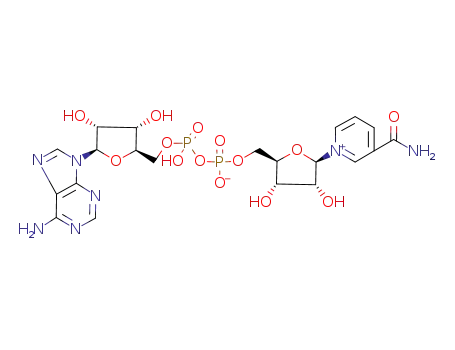
NAD
| Conditions | Yield |
|---|---|
|
With oxygen; coenzyme PQQ; In water; at 30 ℃; Rate constant; pH 6.7; other catalysts, without and with diaphorase;
|
|
|
With oxygen; In water; at 25 ℃; Mechanism; Irradiation; biochemical oscillator with horseradish peroxidase; effect of light, role of methylene blue added; pH 5.10 (sodium acetate buffer);
|
|
|
With oxygen; In water; at 25 ℃; Mechanism; biochemical oscillator with horseradish peroxidase; effects of catalase and superoxide dismutase enzymes added, roles of hydrogen peroxide and methylene blue added; pH 5.10 (sodium acetate buffer);
|
|
|
With α1P2W17VO628-; buffer pH=7; In water; at 20 ℃; under 760 Torr; Mechanism; Rate constant;
|
|
|
With oxygen; diaphorase; pyrroloquinoline quinone; In water; at 30 ℃; pH 6.7;
|
|
|
With mercury dichloride; In water; at 20 ℃; pH=7.4; Further Variations:; Reagents; Kinetics;
|
|
|
With 4-oxo-TPO; Nitrite; Nitrogen dioxide; In phosphate buffer; pH=6.8; Kinetics; ambient temperature;
|
|
|
With poly(aniline)-poly(vinyl sulfonate) modified electrode; In various solvent(s); at 25 ℃; pH=7.0; Kinetics; Electrochemical reaction;
|
|
|
With flavocytochrome P450 BM3 wild type; In various solvent(s); at 15 ℃; pH=7.0; Further Variations:; Reagents; Enzyme kinetics;
|
|
|
Fe3O4-[3-(2-aminoethyl)aminopropyl]trimethoxysilane-PQQ; In various solvent(s); pH=7.0; Kinetics; Electrochemical reaction;
|
|
|
With poly(aniline)-poly(acrylate) film; In various solvent(s); at 25 ℃; Further Variations:; potentials; film thicknesses; Kinetics; Electrochemical reaction;
|
|
|
With sfnoxK2 NADH oxidase from Lactobacillus sanfranciscensis; oxygen; In various solvent(s); at 30 ℃; pH=7; Further Variations:; Reagents; Kinetics; Enzyme kinetics; Enzymatic reaction;
|
|
|
With Bacillus subtilis nitroreductase NfrA1; oxygen; ammonium bicarbonate; In water; at 37 ℃; pH=8.4; Enzymatic reaction;
|
|
|
With human recombinant 3β-hydroxysteroid dehydrogenase/Delta 5->4 isomerase type 2; 5-androstenedione; at 27 ℃; pH=7.4; Concentration; Reagent/catalyst; Kinetics; aq. phosphate buffer;
|
|
|
With Thermus thermophilus lactate dehydrogenase A75G mutant; sodium pyruvate; magnesium chloride; at 25 ℃; pH=7.5; Concentration; Reagent/catalyst; Temperature; Kinetics; aq. buffer; Enzymatic reaction;
|
|
|
With sodium hydroxide; C20H24IrN2O3(1+)*0.5O4S(2-); In water; acetonitrile; at 25 ℃; Conversion of starting material;
|
|
|
NADH; With 1-(4-hydroxy-3,5-dimethoxy-phenyl)-ethanone; triethylamine; In aq. acetate buffer; at 30 ℃; for 0.0833333h; pH=7;
With laccase from Myceliophthora thermophila; In aq. acetate buffer; Reagent/catalyst; pH-value; Catalytic behavior; Kinetics; Enzymatic reaction;
|
|
|
With laccase from Myceliophthora thermophilia; oxygen; methylene blue; In aq. buffer; at 30 ℃; pH=7; Reagent/catalyst; Time; Catalytic behavior; Irradiation; Enzymatic reaction;
|
|
|
With [(η5-pentamethylcyclopentadienyl)Ir(2-(4-hydroxyphenyl)pyridine)Cl]; In methanol; aq. phosphate buffer; at 36.84 ℃; for 24h; pH=7.5; Reagent/catalyst; Concentration; Catalytic behavior;
|
|
|
With reduced flavin mononucleotide; In aq. phosphate buffer; at 30 ℃; pH=7; Reagent/catalyst; Wavelength; Catalytic behavior; Schlenk technique; Irradiation;
|
|
|
With [(η5-C5Me4C6H4C6H5)Ir(2,6-diisopropyl-N-(quinolin-2-ylmethylene)aniline)Cl]PF6; In methanol; water; at 24.84 ℃; for 7.5h; Solvent; Reagent/catalyst; Temperature; Time;
|
|
|
With alcohol dehydrogenase from Saccharomyces cerevisiae; acetaldehyde; Enzymatic reaction;
|
|
|
With C32H42ClO3PRuS; In methanol; water; at 24.84 ℃; for 8h; Reagent/catalyst; Temperature; Solvent;
|
|
|
With C37H41ClIrN4(1+)*Cl(1-); In methanol; water; at 24.84 ℃; for 7h; Kinetics;
|
|
|
With [(η5‐1,2,3,4,5‐pentamethyl‐cyclopentadiene)Ir(1,2‐bis(diphenylphosphino)ethane)Cl]hexafluorophosphate; In methanol; water; at 24.84 ℃; for 8h; Catalytic behavior; UV-irradiation;
|
|
|
With 1,4-dithio-D,L-threitol; NADH oxidase immobilized onto PureCube Ni-IDA MagBeads; In aq. buffer; pH=6.5; Catalytic behavior; Kinetics; Enzymatic reaction;
|
|
|
With C33H33ClFeIrN; In methanol; water; at 24.84 ℃; for 8h; Reagent/catalyst; Catalytic behavior;
|
|
|
With Mycobacterium smegmatis carveol dehydrogenase; 5-[(p-hydroxyphenyl)methyl]-4,4-dimethyl-2,3-pyrrolidinedione; Enzymatic reaction;
|
|
|
With oxygen; [(4,4'-di-tert-butyl-2,2'-bipyridine)2Ru(dipyrido[3,2-a:2’,3’-c]phenazine)]Cl2; In water; for 2h; Reagent/catalyst; Catalytic behavior; Irradiation; Sealed tube;
|
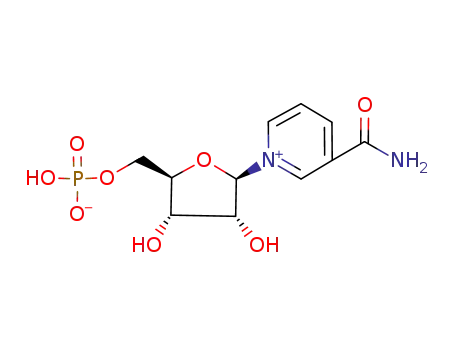
nicotinamide mononucleotide

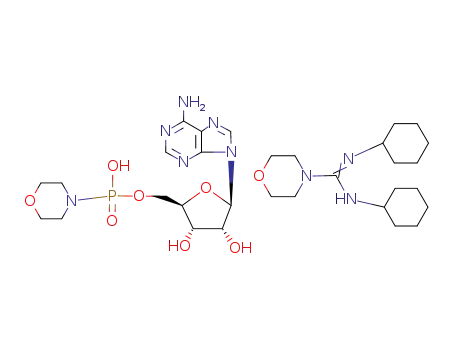
N,N'-dicyclohexyl-4-morpholinecarboxamidinium salt of adenosine-5'-phosphoromorpholidate


NAD
| Conditions | Yield |
|---|---|
|
With magnesium sulfate; manganese(ll) chloride; In formamide; for 16h; Ambient temperature;
|
58% |
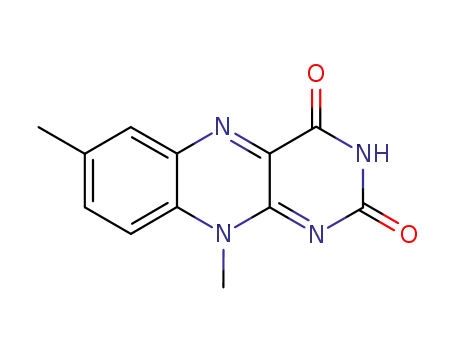
7,10-dimethyl-10H-benzo[g]pteridine-2,4-dione

NADH
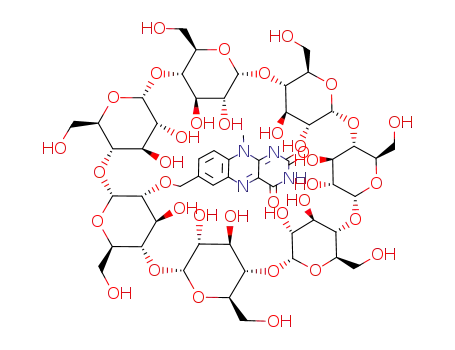
2-<(7α-O-10-methyl-7-isoalloxyazinyl)methyl>-β-cyclodextrin
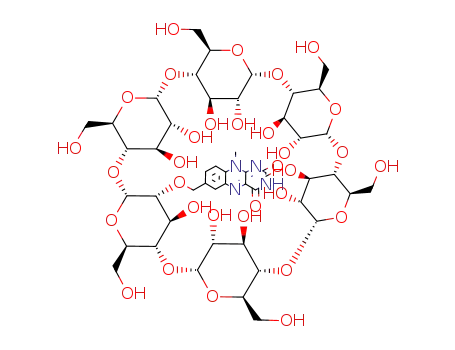
2-<(7α-O-10-methyl-7-isoalloxyazinyl)methyl>-α-cyclodextrin
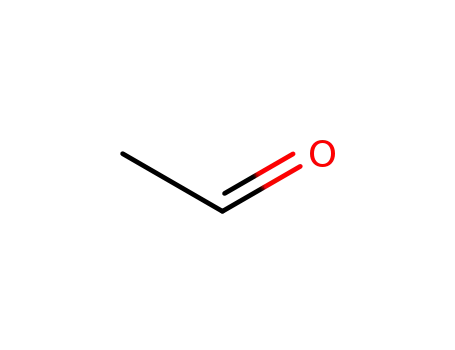
acetaldehyde
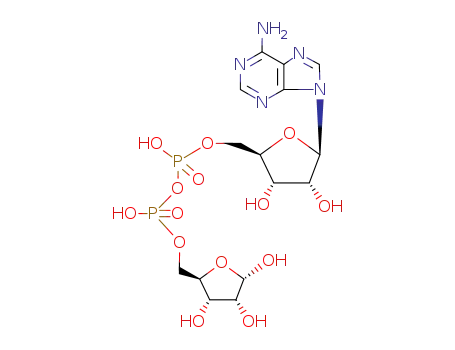
ADP‐ribofuranose
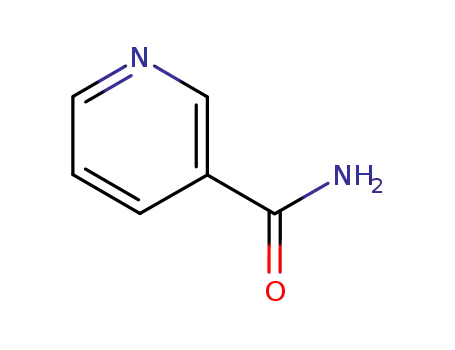
nicotinamide
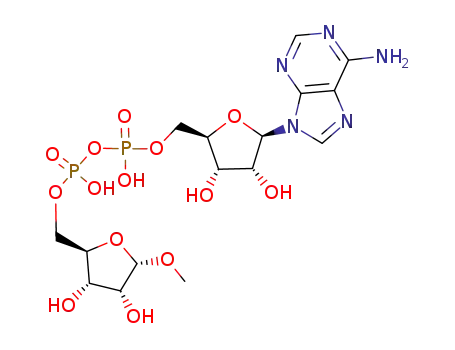
α-1-O-methyl-ADP-ribose