![(3S,6S,9R,12S,15S,23S)-15-[[(2S)-2-acetamidohexanoyl]amino]-9-benzyl-6-[3-(diaminomethylideneamino)propyl]-12-(1H-imidazol-5-ylmethyl)-3-(1H-indol-3-ylmethyl)-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18-hexazacyclotricosane-23-carboxamide](/upload/2023/9/4d5dc114-7b72-4305-a852-9a489aa7e543.png)
CasNo: 121062-08-6
Molecular Formula: C50H69N15O9
Appearance: solid
|
Description |
(3S,6S,9R,12S,15S,23S)-15-[[(2S)-2-acetamidohexanoyl]amino]-9-benzyl-6-[3-(diaminomethylideneamino)propyl]-12-(1H-imidazol-5-ylmethyl)-3-(1H-indol-3-ylmethyl)-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18-hexazacyclotricosane-23-carboxamide is a synthetic analogue of the peptide hormone alpha-melanocyte-stimulating hormone (α-MSH). It's important to note that the use of Melanotan II and similar substances obtained online can be risky due to their unregulated nature and potential adverse effects. The term "Barbie drug" underscores the desire for a tan without sun exposure, but users should be aware of the potential health risks associated with its use. As an unlicensed and untested substance, the safety and efficacy of Melanotan II are not well-established, and caution should be exercised. |
| Systemic Toxicity Concerns | The use of (3S,6S,9R,12S,15S,23S)-15-[[(2S)-2-acetamidohexanoyl]amino]-9-benzyl-6-[3-(diaminomethylideneamino)propyl]-12-(1H-imidazol-5-ylmethyl)-3-(1H-indol-3-ylmethyl)-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18-hexazacyclotricosane-23-carboxamide has been associated with potential systemic toxicity, including sympathomimetic excess, rhabdomyolysis, and renal dysfunction. |
|
Uses |
Melanotan II is used for inducing pigmentation (tanning) of the human skin. Referred to as the 'Barbie drug,' it promises users a rapid tan without extended sun exposure. |
InChI:InChI=1/C50H71N15O10/c1-3-4-16-36(59-29(2)66)44(70)65-41(25-42(67)68)49(75)64-40(24-32-27-55-28-58-32)48(74)62-38(22-30-13-6-5-7-14-30)46(72)61-37(19-12-21-56-50(53)54)45(71)63-39(23-31-26-57-34-17-9-8-15-33(31)34)47(73)60-35(43(52)69)18-10-11-20-51/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25,51H2,1-2H3,(H2,52,69)(H,55,58)(H,59,66)(H,60,73)(H,61,72)(H,62,74)(H,63,71)(H,64,75)(H,65,70)(H,67,68)(H4,53,54,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1
Two subjects had increased pigmentation in the face, upper body and buttock, as measured by quatitative reflectance and by visual perception 1 week after MT-II dosing ended. These results demonstrate that MT-II has tanning activity in humans given only 5 low doses every other day by subcutaneous injection. The recommended single MT-II dose for future Phase I studies is 0.025 mg/kg/day.
The sciatic nerve crush model was used as a paradigm to investigate the neurotrophic properties of melanotan-II. Melanotan-II significantly enhanced the recovery of sensory function following a crush lesion of the sciatic nerve in the rat at a dose of 20 μg kg−1 per 48 h, s.c., but not at a dose of 2 or 50 μg kg−1.
C94H103N14O14PolS

acetic anhydride

Fmoc-(S)-2-aminohexanoic acid

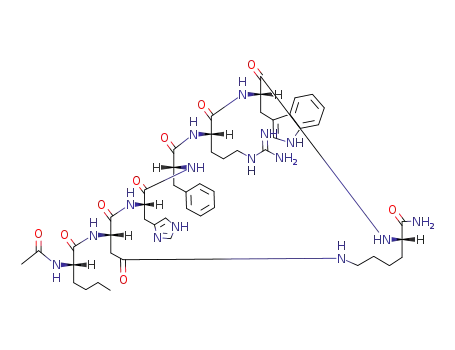
melanotan-II
| Conditions | Yield |
|---|---|
|
C94H103N14O14PolS; With piperidine; benzotriazol-1-ol; In dichloromethane; N,N-dimethyl-d6-formamide; at 20 - 75 ℃; for 0.1h; rink-amide-MBHA resin Microwave irradiation;
Fmoc-(S)-2-aminohexanoic acid; With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine; In dichloromethane; N,N-dimethyl-formamide; at 75 ℃; for 0.0833333h; rink-amide-MBHA resin Microwave irradiation;
acetic anhydride; Further stages;
|
Ac-Nle-Asp-His-D-Phe-Arg-Trp-Lys-NH2


melanotan-II
| Conditions | Yield |
|---|---|
|
With dipotassium hydrogenphosphate; diphenyl phosphoryl azide; In N,N-dimethyl-formamide; 1) 0 deg C, 6 h, 2) r.t., overnight;
|
30% |