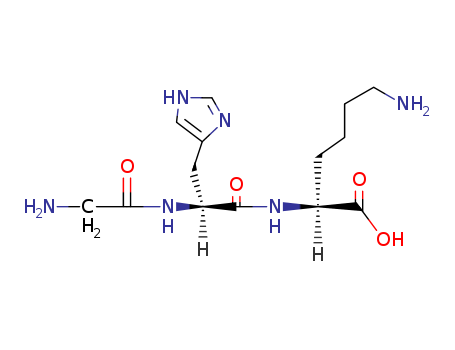
CasNo: 49557-75-7
Molecular Formula: C14H24N6O4
Appearance: White powder
|
Pharmaceutical Applications |
Glycyl-l-histidyl-l-lysine (GHK) is a tripeptide known for its high binding affinity to Cu2+ and its complex role in wound healing. The GHK–Cu(II) complex was isolated from human plasma in the 1970s and it was shown to be an activator for wound healing. GHK–Cu(II) has two main functions: as an anti-inflammatory agent to protect the tissue from oxidative damage after the injury, and as an activator for wound healing itself as it activates the tissue remodelling. The structure of GHK is very similar to that of common drugs used to treat ulcers. After the initial stages of wound healing are activated, such as blood coagulation and neutrophil invasion, a second stage of wound healing begins, which includes the population of GHK at the wound, which has a high affinity to Cu2+. Mast cells, which are located in the skin, secrete GHK, which accumulates Cu2+ and forms the copper complex GHK–Cu(II) and therefore increases the metal–tripeptide concentration at the wound. First, GHK–Cu(II) has an anti-inflammatory effect by protecting the tissue from oxidative damage and by suppressing local inflammatory signals (i.e. cytokine interleukin-1 (IL-1)). Second, GHK–Cu(II) is released into the blood stream and encourages the production of wound macrophages that support the wound repair by removing the damaged tissue and secreting a family of several growth factor proteins. GHK–Cu(II) also hinders fibroblast production of TGF-β-1 and therefore suppresses the scar development. The GHK–Cu(II) complex also stimulates the growth of blood vessels, neurons and elastin, and, in general, supports most processes of wound healing. |
|
|
|
|
Definition |
ChEBI: A tripeptide composed of glycine, L-histidine and L-lysine residues joined in sequence. |
InChI:InChI=1/C14H24N6O4/c15-4-2-1-3-10(14(23)24)20-13(22)11(19-12(21)6-16)5-9-7-17-8-18-9/h7-8,10-11H,1-6,15-16H2,(H,17,18)(H,19,21)(H,20,22)(H,23,24)
A general method for the synthesis of ch...
A solid phase synthesis method for prepa...
A solid phase synthesis method for prepa...
A synthetic peptide which possesses the ...
N-(fluoren-9-ylmethoxycarbonyl)glycine

Fmoc-Lys(tert-butoxycarbonyl)

Fmoc-His(Trt)-OH

glycyl-L-histidyl-L-lysine
| Conditions | Yield |
|---|---|
|
Multistep reaction;
|
40% |

glycyl-L-histidyl-L-lysineamide

glycyl-L-histidyl-L-lysine
| Conditions | Yield |
|---|---|
|
|
N-(fluoren-9-ylmethoxycarbonyl)glycine
Fmoc-Lys(tert-butoxycarbonyl)
Fmoc-His(Trt)-OH