CasNo: 97240-79-4
Molecular Formula: C12H21NO8S
Appearance: white to off-white crystalline powder
|
Broad-spectrum anti-epileptic drugs |
Topiramate (the TPM) is a naturally existing monosaccharide D-fructose sulfide, and together with felbamate, lamotrigine and vigabatrin are current several broad-spectrum anti-epileptic drugs with relatively wide clinical application and can be used to control different types of epilepsy with excellent efficacy and pharmacokinetics. But in cases of being applied to children or fast increase of the amount can cause cognitive impairment and neurotoxicity, and easy to trigger kidney stones. In 1980, scientists had first successfully synthesized tipiramate in the laboratory. It had been applied to patients with epilepsy for the first time in 1986. In 1995, it was approved for entering into the market of UK for the first time. Its basic structure is fructopyranose sulfamate. Unlike other kinds of anti-epileptic drug, TPM has various kinds of anti-epileptic mechanisms including blocking the voltage-dependent sodium channels, enhancing the activity of GABA in the location of the γ-aminobutyric acid A (GABAA) receptor as well as blocking the activity of the AMPA glutamate receptor. In addition, there is still mild effect of carbonic anhydrase inhibitors. Europe and the United States had conducted double-blind, placebo-controlled studies and demonstrated that the adjunctive therapy with topiramate has excellent efficacy, safety and is well tolerated in the treatment of various types of refractory epilepsy. Now it has begun with topiramate monotherapy and has also achieved good results with 62% of patients with epileptic seizures disappearing completely. This product is generally used for the adjuvant treatment of antiepileptic drugs and is effective in treating simple and complex seizures as well as systemic tonic-clonic seizures and can also be used for the treatment of infantile spasms. Features of this product include excellent long-term efficacy, no significant resistance as well as being able to be used alone for antiepileptic purpose. |
|
Indications |
Epilepsy: monotherapy and adjunctive therapy of focal and generalized seizures. Recommendations summarized from NICE (2012) Seizure types: first line (myoclonic seizures), adjunctive (generalized tonicclonic seizures, focal seizures, myoclonic seizures), on referral to tertiary care (tonic/ atonic seizures, absence seizures). Epilepsy types: first line (juvenile myoclonic epilepsy, idiopathic generalized epilepsy, Dravet), adjunctive (juvenile myoclonic epilepsy, epilepsy with generalized tonic- clonic seizures only, idiopathic generalized epilepsy, benign epilepsy with centrotemporal spikes, Panayiotopoulos syndrome, late- onset childhood occipital epilepsy), on referral to tertiary care (absence syndromes), contraindicated (Lennox– Gastaut syndrome). Neurology: migraine prophylaxis |
|
Pharmacological effects |
The major mechanism of Topiramate is through blocking the dispersion of epileptic seizures rather than preventing its occurrence. It has been also found that TPM can exert its efficacy in treating epilepsy through various kinds of mechanisms including: 1, blocking the voltage-dependent sodium channels and thereby reducing the duration of epileptic discharges and the number of action potentials generated during each discharge. 2, antagonizing kainate/AMPA--glutamate receptors. 3, enhancing the GABA activity in the non-benzodiazepine position of the GABA receptor. 4, mildly inhibiting carbonic anhydrase. 5, blocking the T-type calcium channels. 6, block the activity of the excitatory neurotransmitter of the central nervous AMPA glutamate receptor. Recent studies have shown that blocking L-type high-voltage-dependent calcium channels may be one of the most important mechanisms of the action of antiepileptic topiramate. Topiramate (TPM) can be used to prevent the animal epileptic seizures induced by maximum electroshock seizure test (MES) but has no effect on the chemical drug-induced epileptic seizure as well as can’t be used to prevent the occurrence of the epileptic seizures. Electrode physiological studies on the cultured hippocampal neurons have shown that 10μmol/ml topiramate can reduce the incidence of neural ignited spontaneously epileptic seizures and action potentials while 20μmol/ml of TPM can reduce the frequency of action potential firing. The antiepileptic effect of topiramate may also be related to its effect on increasing the GABA-induced influx of the chloride ions. Similar to benzodiazepine, TPM is also capable of increasing the GABA induced penetration of chloride particles through the cultured cell membrane. In addition to affecting the flow of chloride ions, TPM also increase the frequency of CABA’s activation of GABAA receptor. However, the activation is not through the interaction of the GABA binding site or benzodiazepine binding site. Topiramate has also been found to have mild inhibitory effect on the two carbonic anhydrase isozymes: carbonic anhydrase II and carbonic anhydrase IV. The above information is edited by the lookchem of Dai Xiongfeng. |
|
Pharmacokinetics |
Topiramate is a white crystalline powder with bitter taste and is easily soluble in alkaline solution. Its saturated solution has a pH of 6.3. It can be rapidly absorbed after oral administration with achieving the average Cmax = 1.5 μg/ml within 2 to 3 hours (Tmax). Food had no significant effect on the bioavailability of topiramate with the oral absorption averaged on about 81%. There is 13% to 17% of topiramate binding to the plasma proteins with the average volume of distribution being 0.55~0.80L/kg. Upon single oral administration of a dosage of 100~400mg, it exhibits a linear drug-metabolism property. Patients with normal renal function can have it reach steady-state plasma concentrations in 4 to 8 days. Oral administration of 50 mg and 100 mg 2 times per day has the average T1/2 of approximately 21 hours. Oral administration of 100~400mg with 2 times per day together with taking phenytoin or carbamazepine can increase the plasma concentrations of the latter two drugs in positive dose-dependent relationship. Therefore, such patients should be subject to close observation on the adverse reactions and monitoring of the drug plasma concentrations when necessary. Instead, carbamazepine and phenytoin sodium can reduce the plasma concentration of topiramate. Therefore we need to adjust the dose based on efficacy and adverse reactions. Valproate doesn’t significantly affect the plasma concentration of topiramate. Only about 20% of the topiramate can be subject to metabolism in its prototype. When used in combination with antiepileptic drug for treatment, about 50% of the topiramate is converted by metabolic enzymes. The six kinds of topiramate metabolites produced by the body all have no obvious anticonvulsant activity. 80% of the prototype topiramate in the body as well as its metabolites are subject to renal clearance. Oral administration of 50~100mg for 2 times per day gives the average renal clearance rate being about 18ml/min. Patients of renal impairment or hepatic injury has a decreased plasma clearance and renal clearance rate and the time for reaching steady state plasma concentration may take 10 to 15 days. Usually elderly patients have their plasma clearance rate be unchanged. |
|
Dosage |
It acts as adjuvant drugs for the treatment of partial epileptic seizure with or without secondary systemic seizure. It is preferably to start administrating it from a low dose and gradually increase to the effective dose. Do not crush the tablets. For adults, it is recommended to take 50 mg per night during the first wee; take 50 mg at both day and night during the second week; take 50 mg in the morning and 100 mg in the evening; take 100 mg at both day and night during the fourth week; take 100 mg at day and take 150 mg at night in the fifth week; take 150 mg at both day and night during the sixth week; take 150 mg at day and 200 mg at night during the seventh week; take 200 mg at both day and night at the eighth week. Maintenance dose: 400mg/d. For children of 2 to 16 years old, the recommended dosage is 5~9mg/kg daily and divided into 2 times. The dose should be adjusted to 25mg (day 1~3 mg/kg) at night during the first week and then add 1~3mg/kg every day at the interval of 1 to 2 weeks with administration in 2 times until reaching the optimal clinical efficacy. Dose titration Epilepsy Monotherapy 25 mg nocte for 7 days, then increased by 25– 50 mg every 7– 14 days; usual maintenance 100– 200 mg daily divided into two doses (max 500 mg daily, although doses of 1000 mg daily have been used for refractory epilepsy). Adjunctive therapy 25– 50 mg nocte for 7 days then increased by 25– 50 mg every 7– 14 days; usual maintenance 200– 400 mg daily divided into two doses (max 400 mg daily). In case of a missed dose, take the next dose; do not take an extra tablet to make up for the missed one. |
|
Clinical evaluation |
33 cases of patients of epilepsy apply monotherapy or combination treatment with other antiepileptic drugs with the initial dose of 25mg, qd; the other 40 patients were subject to carbamazepine treatment as control. The effective rate of the topiramate treatment group was 93.9% which is significantly higher than that of the carbamazepine treatment group 77.5% (P <0.05); topiramate monotherapy has a better efficacy than combination therapy. In preclinical tests of the AEDs development of the National Institutes of Health (NIH), researchers had studied the efficacy of topiramate as adjuvant therapy in the treatment of adult epilepsy patients. 41% of patients treated with topiramate had their seizure times decreased> 50% while the value in the placebo group was only 10%; 19% of patients treated with topiramate get the seizure times decreased> 75% (the value is only 3% in the placebo group). |
|
Cautions |
Patients with acute porphyrias. Patients with risk factors for metabolic acidosis. Patients with risk factors for nephrolithiasis (ensure adequate hydration). |
|
Adverse reactions and precautions |
1, since topiramate is often used in combination with other anti-epileptic drugs, it is therefore difficult to distinguish which drug or several drugs are related to the adverse reactions. The most frequently reported adverse reactions drug are symptoms associated with the central nervous system including ataxia, impaired attention, confusion, dizziness, fatigue, paresthesia, somnolence and abnormal thinking. This is potential risk factors for patient in driving or operating machinery. Common adverse reactions include anxiety, forgetting, loss of appetite, aphasia, depression, diplopia, mood swings, nausea, nystagmus, verbal expression disorder, taste perversion, abnormal vision and weight loss. Occasionally there are reports of renal stone disease. Drinking lots of water during the treatment can reduce the risk factors. Patients known allergic to the chemicals should be disabled. 2, similar to other anti-epileptic drugs, we should discontinue it gradually so that the possibility of increased seizure frequency can be reduced to minimum. In clinical trials, the reduced amount weekly is 100mg/day. 3, same as with other antiepileptic drugs, animal experiments confirmed that topiramate has teratogenic effects. 4, upon acute overdose, if ingested just now, you should immediately adopt gastric tube induced gastric emptying or induced vomiting for gastric emptying for rescue. Activated carbon does not adsorb topiramate, therefore it is not recommended to apply it upon overdose. Apply hemodialysis if necessary. 5, when using in combination phenytoin, we should monitor with the phenytoin plasma concentrations. Phenytoin and carbamazepine can reduce the plasma concentration of this product. For patients taking digoxin, when adding or discontinuing this product, pay attention to monitoring the digoxin plasma concentration. When topiramate is used in combination with oral contraceptives, the efficacy of the contraceptive may be reduced. You may need to adjust the dose of this product when added with hydrochlorothiazide. It is not recommended to administer together with alcohol or other central nervous system depressants. For patients being subject to metformin treatment, if increasing or stopping the treatment of this product, it should be closely monitored of the diabetic condition. Upon being used combination with pioglitazone, we should pay attention to the control of the diabetes disease. Being used in combination with other drugs which can lead to kidney stones can increase the risk of kidney stones. Overdose may cause convulsions, drowsiness, speech disorder, blurred vision, diplopia, mental impairment, lethargy, ataxia, stupor, hypotension, abdominal pain, dizziness and depression. Topiramate could result in severe metabolic acidosis. |
|
Interactions |
With AEDs Carbamazepine and phenytoin decrease the plasma concentration of topimarate. Therefore, the addition or withdrawal of carbamazepine and phenytoin to topiramate therapy may require an adjustment in dosage of the latter: this should be done by titrating to clinical effect. The addition of topiramate to phenytoin may result in an increase of plasma concentrations of phenytoin, possibly due to inhibition of a specific enzyme polymorphic isoform (CYP2C9). Therefore, any patient on phenytoin showing clinical signs or symptoms of toxicity should have phenytoin levels monitored. With other drugs Nil. With alcohol/food Concomitant administration of topiramate and alcohol (or other CNS depressant drugs) has not been evaluated in clinical studies. it is recommended that topiramate not be used concomitantly with alcohol or other CNS depressant drugs. There are no specific foods that must be excluded from diet when taking topiramate. |
|
Special populations |
Hepatic impairment Use with caution in moderate to severe impairment, as topiramate clearance may be reduced. Renal impairment In patients with impaired renal function topiramate should be administered with caution as the plasma and renal clearance of topiramate are decreased. Subjects with known renal impairment may require a longer time to reach steady- state at each dose. Half of the usual starting and maintenance dose is recommended. Pregnancy Clinical data from pregnancy registries indicate that infants exposed to topiramate monotherapy have an increased risk (three- fold for topiramate monotherapy) of major congenital malformations (particularly cleft lip/ palate, hypospadias, and anomalies involving various body systems) following exposure during the first trimester (increased risk of teratogenic effects associated with the use of AEDs in combination therapy). Clinical data from pregnancy registries indicate that infants exposed to topiramate monotherapy have a higher prevalence of low birth weight (<2500 g) compared with a reference group. It is therefore recommended that women of child- bearing age use highly effective contraception and consider alternative therapeutic options. In case of administration during the first trimester, careful prenatal monitoring should be performed. The dose of topiramate should be monitored carefully during pregnancy and after birth, and adjustments made on a clinical basis. It is recommended that the foetal growth is monitored. Although the excretion of topiramate in human milk has not been evaluated in controlled studies, limited observations in patients suggest an extensive excretion of topiramate into breast milk. Therefore, the options of suspending breastfeeding or discontinuing/ abstaining from topiramate therapy should be carefully weighed up. |
|
Psychiatric use |
There is some evidence that topirmate may be effective in the treatment of depression, either as monotherapy or as adjunctive treatment. The findings of initial reports suggesting that topiramate can be effective in the treatment of bipolar disorder and post- traumatic stress disorders have not been confirmed by the results of randomized controlled trials. Preliminary data suggest some efficacy in Tourette syndrome, obsessive- compulsive disorder, eating disorders (binge eating), behavioural and psychological symptoms of dementia, alcohol and cocaine dependence. In late 2012, topiramate was approved by the USA FDA in combination with phentermine for weight loss: this is a clinically significant effect in patients with behavioural problems, as psychopharmacological treatment is often associated with metabolic dysfunction and weight gain. |
|
Manufacturing Process |
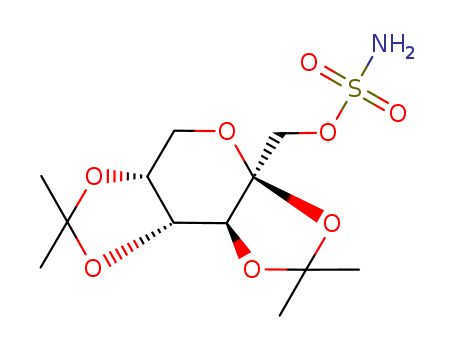
To a cold solution (-4°C) of 2,3:4,5-di-O-isopropylidene-β-fructopyranose (75 g, 0.29 mol) in DMF (725 ml) was added 50% oily sodium hydride (16.34 g,0.34 mol). After stirring for 90 min, sulfamoyl chloride (54.9 g, 0.48 mol) was added and the stirring continued for an additional 3.5 h at that temperature. The reaction mixture was poured into cold water and extracted with toluene. The organic layer was dried (Na2SO4) and the solvents removed under vacuum to give a syrup which crystallized immediately. Recrystallization from ethylacetate/hexane gave pure 2,3:4,5-bis-O-(1-methylethylidene)-β-D_x0002_fructopyranose sulfamate, melting point 125°-126°C. |
|
Biological Functions |
Topiramate is most useful in patients with generalized tonic–clonic seizures and those with partial complex seizures. Topiramate causes a higher incidence of CNSrelated side effects (primarily cognitive slowing and confusion) than other AEDs. It does not appear to cause a significant incidence of rashes or other hypersensitivity reactions; however, a significantly higher incidence of kidney stones has been observed in persons receiving topiramate than in a similar untreated population. |
|
Biological Activity |
Anticonvulsant. Antagonizes GluR5 kainate receptors (IC 50 = 0.46 μ M), acts as a positive allosteric modulator of GABA A receptor-mediated currents, inhibits Na v channels (IC 50 = 48.9 μ M) and inhibits L-type Ca 2+ channels. Also inhibits carbonic anhydrase (CA) (K i values are 0.1 and 0.2 μ M at rat CA II and CA IV respectively), which lowers intracellular neuronal pH. |
|
Biochem/physiol Actions |
Kainate GluR5 receptor antagonist; anticonvulsant. |
|
Mechanism of action |
The mechanism of action for topiramate is unknown, but several actions are thought to contribute to its AED activity. It blocks repetitive firing by acting on sodium channels, may enhance GABAA-mediated chloride flux, and appears to be an antagonist at the AMPA and KA receptors, thus blocking the effect of glutamate. In addition, recent evidence suggests inhibition of L-type calcium currents. |
|
Side effects |
Common CNS side effects associated with topiramate therapy include drowsiness, dizziness, impaired concentration and memory, speech and language difficulties, and confusion. These effects develop during the first weeks of therapy and may decline over time. Acute closed-angle glaucoma caused by topiramate requires immediate evaluation. Only rare hepatic or bone marrow effects have been noted thus far; however, an increased incidence of renal stones is troublesome and probably related to the drug's activity as a carbonic anhydrase inhibitor, reducing citrate excretion and increasing urinary pH. Use of additional carbonic anhydrase inhibitors, a ketogenic diet, or a family history of nephrolithiasis may be considered as contraindications for using topiramate. Topiramate is not devoid of potential interaction properties: It induces CYP3A4 and inhibits CYP2C19, thus significantly increasing plasma phenytoin levels. Topiramate also may decrease the effectiveness of oral contraceptives. |
|
Veterinary Drugs and Treatments |
Topiramate may be useful for treating seizures in dogs, particularly partial seizure activity. It may also be of benefit in treating cats, but little information is available. |
|
in vitro |
in principal neurons of the rat basolateral amygdala, low concentrations of topiramate selectively inhibited pharmacologically isolated excitatory synaptic currents mediated by kainate receptors with the glur5 subunit with an ic50 value of 0.5 μm. topiramate also partially depressed predominantly ampa-receptor-mediated epscs with lower efficacy [1]. in dissociated neocortical slices, low concentrations of tpm (25–30 μm) slightly inhibited the persistent fraction of na+ current and reduced the na+-dependent long-lasting action potential shoulders evoked in layer v pyramidal neurons after ca2+ and k+ current blockade. tpm (100 μm) had no effects on the voltage dependence of activation but induced a leftward shift of the steady-state inaf inactivation curve [3]. |
|
in vivo |
tpm treatment significantly improved the 24-h neurological deficit scores (high dose, 1.17 ± 0.41; low dose, 1.75 ± 0.5; p < 0.05 for both doses). the percentage of infarct volume (low dose, 22.9 ± 8.9%, p = 0.002; high dose 7.6 ± 3.4%, p < 0.001) reduced when compared with the controls (infarct size, 54.2 ± 9.0%; neurobehavior score, 2. 67 ± 0.52). higher dose of tpm induced more neuroprotection than that of lower dose (p < 0.05). in a rat model of focal ischemia, treatment with tpm 2 h after mca embolization resulted in neuroprotective effect in a dose- and use-dependent manner [2]. topiramate (25-100 mg/kg, i.p.) dose-dependently elevated the threshold for clonic seizures induced by infusion of a selective agonist of glur5 kainate receptors atpa [4]. topiramate (i.p) effectively suppressed acute seizures induced by perinatal hypoxia in a dose-dependent manner with an ed50 of 2.1 mg/kg [5]. topiramate (20 and 40 mg/kg i.p.) dose-dependently inhibited both tonic and absence-like seizures. in dba/2 mice, topiramate inhibited sound-induced seizures with ed50 of 8.6 mg/kg (p.o) [6]. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antidepressants: antagonism of anticonvulsant effect; avoid with St John’s wort. Antiepileptics: concentration reduced by fosphenytoin, phenytoin, carbamazepine and possibly phenobarbital; increases fosphenytoin and phenytoin concentration; reduces concentration of perampanel; hyperammonaemia and CNS toxicity reported with valproate. Antimalarials: mefloquine antagonises anticonvulsant effect. Antipsychotics: anticonvulsant effect antagonised. Oestrogens and progestogens: reduced contraceptive effect. Orlistat: possibly increased risk of convulsions. Ulipristal: reduced contraceptive effect - avoid. |
|
Metabolism |
Topiramate is not extensively metabolised (~20%) in healthy volunteers. It is metabolised up to 50% in patients receiving enzyme-inducing drugs. Six metabolites formed through hydroxylation, hydrolysis and glucuronidation have been identified but have little activity. It is eliminated chiefly in urine, as unchanged drug and metabolites. |
|
references |
[1] gryder d s, rogawski m a. selective antagonism of glur5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons[j]. the journal of neuroscience, 2003, 23(18): 7069-7074.[2] yang y, shuaib a, li q, et al. neuroprotection by delayed administration of topiramate in a rat model of middle cerebral artery embolization[j]. brain research, 1998, 804(2): 169-176.[3] taverna s, sancini g, mantegazza m, et al. inhibition of transient and persistent na+ current fractions by the new anticonvulsant topiramate[j]. journal of pharmacology and experimental therapeutics, 1999, 288(3): 960-968.[4] kaminski r m, banerjee m, rogawski m a. topiramate selectively protects against seizures induced by atpa, a glur5 kainate receptor agonist[j]. neuropharmacology, 2004, 46(8): 1097-1104.[5] koh s, jensen f e. topiramate blocks perinatal hypoxia‐induced seizures in rat pups[j]. annals of neurology, 2001, 50(3): 366-372.[6] nakamura j, tamura s, kanda t, et al. inhibition by topiramate of seizures in spontaneously epileptic rats and dba/2 mice[j]. european journal of pharmacology, 1994, 254(1-2): 83-89. |
|
Definition |
ChEBI: A hexose derivative that is 2,3:4,5-di-O-isopropylidene-beta-D-fructopyranose in which the hydroxy group has been converted to the corresponding sulfamate ester. It blocks voltage-dependent sodium channe s and is used as an antiepileptic and for the prevention of migraine. |
|
Brand name |
Topamax |
|
General Description |
TPM is a sulphamate-substituted monosaccharide, a derivativeof the naturally occurring sugar D-fructose thatexhibits broad and potent AED actions at both glutamateand GABA receptors.19 It has good oral bioavailability of85% to 95%, most likely resulting from its structural similarityto D-glucose. Thus, it may be actively transportedinto the brain by the D-glucose transporter. (Recall thatD-fructose and D-glucose have identical stereochemistry atmany of their chiral centers.) Only about 20% of the drugis eliminated by hepatic metabolism (CYP2C19), the remainingdrug is excreted unchanged by the kidneys.57 Thesulphamate ester is hydrolyzed by sulfatases to the correspondingprimary alcohol, which is further oxidized to thecorresponding carboxylic acid. Even though there are noreports of significant interactions between TPM and otherAEDs, TPM is said to have a weak carbonic anhydrase inhibitoryactivity because of the presence of the sulphamatemoiety. Thus, concomitant use of TPM with other carbonicanhydrase inhibitors should be avoided.57 The exact mechanismof actions are still unknown, but TPM appears toblock glutamate release, antagonize glutamate kainicacid/AMPA receptors, and increase GABAergic transmissionby binding to a site distinct from BZDs or barbiturateson the GABAA receptor complex. |
InChI:InChI=1/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9?,12+/m0/s1
Sulfamates and sulfamides are most often...
The invention belongs to the technical f...
The invention belongs to the technical f...
The invention discloses a preparation me...
N-benzyloxycarbonyl-2,3: 4,5-di-O-(1-methylethylidene)-β-D-fructopyranosaminosulfonates potassium salt

topiramate
| Conditions | Yield |
|---|---|
|
With palladium on activated charcoal; In methanol; at 20 ℃; for 3h; under 9000.9 Torr;
|
83% |
C15H26N2O8S

topiramate
| Conditions | Yield |
|---|---|
|
With hydrazine hydrate; In ethanol; at 20 ℃; for 1h;
|
90% |
2,3;4,5-di-O-isopropylidene-β-D-fructopyranose
2,3-O-(1-Methylethylidene)-β-D-fructopyranose 1-sulfamate
orthoformic acid triethyl ester
ethyl trifluoroacetaldehyde hemiacetal
N-(2-methoxycarbonylphenyl)carbonyl-2,3:4,5-bis-O-(1-methylethylidene)-β-D-fructopyranose sulfamate
2,3:4,5-bis-O-(1-methylethylidene)-β-D-fructopyranose methylsulfamate