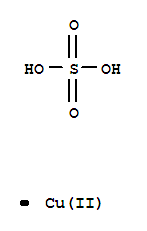
CasNo: 7758-98-7
Molecular Formula: CuSO4
Appearance: Bluish crystalline powder
|
Chemical Description |
Copper(II) sulfate is a blue salt used as a fungicide and herbicide. |
|
Air & Water Reactions |
Soluble in water. |
|
Reactivity Profile |
Anhydrous Cupric sulfate serves as a weak oxidizing agent. Causes hydroxylamine to ignite. Gains water readily. The hydrated salt is vigorously reduced by hydroxylamine [Mellor 8:292(1946-1947)]. Both forms are incompatible with finely powdered metals. Both are incompatible with magnesium, corrode steel and iron, may react with alkalis, phosphates, acetylene gas, hydrazine, or nitromethane, and may react with beta-naphthol, propylene glycol, sulphathiazole and triethanolamine if the pH exceeds 7 . Both act as acidic salts, corrode metals and irritate tissues. |
|
Hazard |
Toxic; highly irritant. |
|
Health Hazard |
Workers who accidentally ingest copper sulfate experience abdominal pain and cramps, burning sensation, corrosive effects, nausea, vomiting, loose bowel movement, and a metallic taste. Exposures to copper sulfate by ingestion or skin absorption cause severe irritating effects to the eyes and skin The aerosol is irritating to the respiratory tract, and effects on the blood, kidneys and liver result in hemolytic anemia, kidney impairment, liver impairment, and shock or collapse. At large doses, accidental intake of copper sulfate causes renal failure, comatose, and even death. Long-term exposure to copper sulfate may lead to liver damage, lung diseases, and decreased female fertility. |
|
Trade name |
AGRITOX?; BASICOP?; BCS COPPER FUNGICIDE?; BSC FLOWABLE?[C]; COPSIN?; CP BASIC SULFATE?; CUPROFIX?; FUNGI-SPERSE II[C]; SULTRACOB?; TNCS? 53; TRIANGLE? |
|
Safety Profile |
A human poison by ingestion. An experimental poison by ingestion, subcutaneous, parenteral, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: gastritis, Qarrhea, nausea or vomiting, damage to kidney tubules, and hemolysis. Questionable carcinogen with experimental tumorigenic data. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. Reacts violently with hydroxylamine, magnesium. See also COPPER COMPOUNDS and SULFATES. When heated to decomposition it emits toxic fumes of SOx |
|
Potential Exposure |
Copper sulfate is used as intermediate and wood preservative; also used in production of copper compounds; to detect and to remove trace amounts of water from alcohols and organic compounds; as a fungicide and algicide; in veterinary medicine and others. |
|
storage |
Workers should keep copper sulfate stored in a cool, dry area with suffi cient ventilation. It should be kept away from alkalis, magnesium, ammonia, acetylene, and sodium hypobromite. |
|
Shipping |
UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required. |
|
Purification Methods |
After adding 0.02g of KOH to a litre of nearly saturated aqueous solution of the sulfate, it is left for two weeks, then the precipitate is filtered on to a fibreglass filter with pore diameter of 5-15 microns. The filtrate is heated to 90o and allowed to evaporate until some CuSO4.5H2O crystallises out. The solution is then filtered hot and cooled rapidly to give crystals which are freed from mother liquor by filtering under suction [Geballe & Giauque J Am Chem Soc 74 3513 1952]. Alternatively crystallise the sulfate from water (0.6mL/g) between 100o and 0o. The pentahydrate is slowly efflorescent, losing 2H2O at 30o, two more H2O are lost at 110o and a white anhydrous powder (dessicant) is obtained on heating above 250o. |
|
Incompatibilities |
Aqueous solution is an acid. May form explosive materials on contact with acetylene and nitromethane. Incompatible with strong bases; hydroxylamine, magnesium; zirconium, sodium hypobromite, hydrazine. |
|
Waste Disposal |
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill Add soda ash to waste CuSO4 solution; let stand 24 hours. Decant and neutralize solution before flushing to sewer. Landfill sludge. |
|
Precautions |
During handling and use of copper sulfate, students and occupational workers should wear safety glasses and should not breathe the material in powder form. Copper sulfate is an environmental pollutant and must be carefully incorporated when used in its varied applications. Workers should wear protective clothing, goggles, impermeable gloves, and rubber boots to avoid skin contact |
|
Aquaculture |
Copper sulfate is widely used in aquaculture to treat infectious diseases caused by parasitic protozoa and crustaceans, such as Cryptobia sp., Trichodina sp., Chilodonella sp., and Sinergasilus sp. |
|
Umbilical Granuloma Treatment |
Copper sulfate (CuSO4) is utilized for chemical cauterization in the treatment of umbilical granuloma, although it can cause superficial skin burns. An alternative treatment using table salt (NaCl) osmotically dehydrates granulation tissue, leading to necrosis. |
|
Historical Uses and Toxic Properties |
Copper sulfate has been historically used for wound debridement due to its antiseptic properties and as an emetic agent in intoxications. It is found in certain dental cements and intrauterine devices. While poisoning is rare, it can lead to gastrointestinal disease, with oral ingestion being the most common route. |
|
Marine Parasite Control |
Copper sulfate is employed to combat marine parasites like Cryptocaryon irritans, a harmful parasite in mariculture. |
|
Biotoxicity Studies |
Copper sulfate is studied as a biotoxin, particularly its impact on seed germination and microbial growth inhibition. It has shown little impact on seed germination rates but is effective against oomycete pathogens like Phytophthora and Halophytophthora, suggesting its use as an antimicrobial treatment. |
|
Essential Trace Element |
Copper (Cu) in the form of copper sulfate is essential for living organisms' efficient functioning. It acts as a potent oxidant and has a history of agricultural use as a fungicide, bactericide, and pesticide, particularly against smaller animals like snails. The toxicity of copper depends on factors such as physical form, dosage, and exposure period, with potential effects on embryos and growth rates. |
|
Definition |
A compound prepared as the hydrate by the action of dilute sulfuric acid on copper( II) oxide or copper(II) carbonate. On crystallization, blue triclinic crystals of the pentahydrate (blue vitriol, CuSO4.5H2O) are formed. Industrially copper(II) sulfate is prepared by passing air through a hot mixture of dilute sulfuric acid and scrap copper. The solution formed is recycled until the concentration of the copper(II) sulfate is sufficient. Copper(II) sulfate is readily soluble in water. The monohydrate (CuSO4.H2O) is formed at 100°C and the anhydrous salt at 250°C. Anhydrous copper( II) sulfate is white; it is extremely hygroscopic and turns blue on absorption of water. It decomposes on heating to give copper(II) oxide and sulfur(VI) oxide. Copper(II) sulfate is used as a wood preservative, a fungicide (in Bordeaux mixture), and in the dyeing and electroplating industries. |
|
General Description |
A white or off-white solid. Melting point 200°C with decomposition. Non-combustible. |
|
Agricultural Uses |
Fungicide, Algaecide, Bactericide, Herbicide, Molluscicide: Copper sulfate is a fungicide used to control bacterial and fungal diseases of fruit, vegetable, nut, and field crops. These diseases include mildew, leaf spots, blights, and apple scab. It is used as a protective fungicide (Bordeaux mixture) for leaf application and seed treatment. It is also used as an algaecide and herbicide, and to kill slugs and snails in irrigation and municipal water treatment systems. It has been used to control Dutch elm disease. It is available as a dust, wettable powder, or liquid concentrate. Used as a fungicide and algaecide, in veterinary medicine and others. Copper sulfate is also used todetect and to remove trace amounts of water from alcohols and organic compounds. |
|
Industrial uses |
Copper sulfate (CuSO4·5H2O) is widely used as an activator for sphalerite, pyrite, pyrrhotite and other sulfides during processing of base metal ores. During flotation of some silicate minerals, copper sulfate is used as depressant, e.g. zirconium.In manufacturing copper sulfate, sulfuric acid and scrap copper metal are used. The process is based on the oxidation of metal and dissolution with H2SO4 according to the following reaction: 4Cu + O2 = 2Cu2O Cu2O + H2SO4 = CuSO4 + H2O 2Cu2SO4 + 2H2SO4 + O2 = 4CuSO4 + 2H2O Usually, in mineral processing applications, copper sulfate is delivered in crystal form. |
InChI:InChI=1/Cu.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2