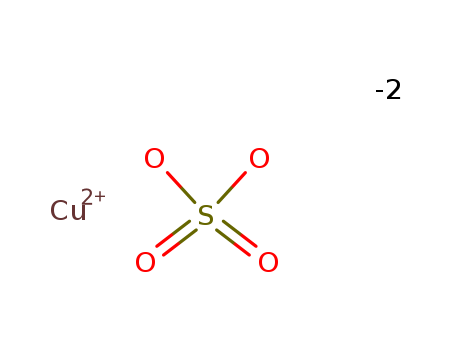
CasNo: 7758-99-8
Molecular Formula: CuSO4.5(H2O)
Appearance: Blue crystalline granules or powder
|
Air & Water Reactions |
Slowly effloresces in air. Water soluble. |
|
Reactivity Profile |
Copper sulfate pentahydrate can be dehydrated by heating. Serves as a weak oxidizing agent. Causes hydroxylamine to ignite. Gains water readily. The hydrated salt is vigorously reduced by hydroxylamine [Mellor 8:292(1946-1947)]. Both forms are incompatible with finely powdered metals. Both are incompatible with magnesium, corrode steel and iron, may react with alkalis, phosphates, acetylene gas, hydrazine, or nitromethane, and may react with beta-naphthol, propylene glycol, sulphathiazole and triethanolamine if the pH exceeds 7 . Both act as acidic salts, corrode metals and irritate tissues. |
|
Fire Hazard |
Literature sources indicate that Copper sulfate pentahydrate is nonflammable. |
|
Definition |
ChEBI: Copper(II) sulfate pentahydrate is the pentahydrate of copper(2+) sulfate. A bright blue crystalline solid. It is a hydrate and a metal sulfate. It contains a copper(II) sulfate. |
|
Application |
Copper(II) sulfate pentahydrate has been used:as an additive in trace element solution preparation in solid glucose minimal media.as a component of adamsII solution in Pneumococcal media.in the preparation of alginate gel for drug encapsulation.Copper(II) sulfate is an inorganic Lewis acid commonly used to promote acid catalyzed organic reactions. It is used as a reagent for the synthesis of copper carbenoids. It can also act as an effective redox catalyst in combination with other mixed oxidizing systems.Copper Sulfate Pentahydrate Fine Crystals serve as the main ingredient for manufacturing Bordeaux and Burgundy mixtures that are used as algaecides, both on the farm and to ensure safe water supplies. Bordeaux mixtures are also employed to adjust and maintain copper deficient soils to optimum levels. |
|
General Description |
Blue crystalline granules or powder. Melting point 110°C (with decomposition). Non-combustible. Nauseating metallic taste. Odorless. White when dehydrated. |
InChI:InChI=1/Cu.H2O4S.5H2O/c;1-5(2,3)4;;;;;/h;(H2,1,2,3,4);5*1H2/q+2;;;;;;/p-2
Copper diethyldithiocarbamate, cadmium d...
Thermogravimetry was used in the study o...
Reactions of (bipy=2,2'-bipyridyl; L=PP...
Results of the experimental investigatio...
The crystal structure of copper sulfate ...
In this paper, the vibrational spectra o...
A thermobalance for teaching purposes wa...
Simultaneous Differential Thermal Analys...
Difference in the isotopic partition at ...
The process of salt roasting appears to ...
Hydrothermal reaction of CuCN, K3[Fe(CN)...
A novel and direct approach to alkyl/ary...
The present invention relates to isolate...
Without using any surfactant or template...
copper(I) sulfide

sodium chloride

disulfur dichloride

copper oxychloride

copper(II) sulfate

copper dichloride
| Conditions | Yield |
|---|---|
|
With oxygen; byproducts: Na2SO4, SO2; information about the react. eqs. in detail and about dependence on temp.;
|
|
|
With oxygen; byproducts: Na2SO4, SO2; information about the react. eqs. in detail and about dependence on temp.;
|
copper iron disulfide

iron(II) oxide

iron(III) oxide

copper(II) sulfate
| Conditions | Yield |
|---|---|
|
With oxygen; byproducts: SO2; heating under the ignition temp. and following roasting at 595°C; information about the react. eqs. in detail;
|
|
|
With oxygen; byproducts: SO2; heating under the ignition temp. and following roasting at 595°C; information about the react. eqs. in detail;
|
sulfuric acid
water
ozone
sodium sulfate
3-HYDROXYPYRIDINE
4-Chloropyridine
phenyl benzyl ketone
indole