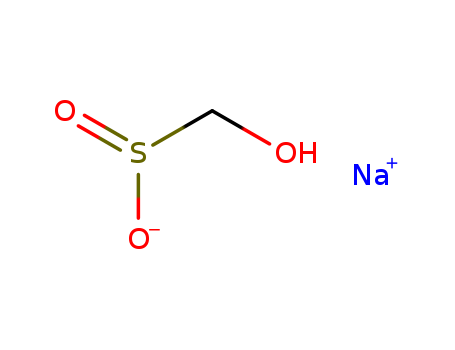
CasNo: 149-44-0
Molecular Formula: CH3NaO3S
Appearance: solid
|
Production Methods |
Sodium formaldehyde sulfoxylate is manufactured from sodium dithionate and formaldehyde in water. |
|
Flammability and Explosibility |
Nonflammable |
|
Pharmaceutical Applications |
Sodium formaldehyde sulfoxylate is a water-soluble antioxidant and is generally used as the dihydrate. It is used in the formulation of injection products at a level of up to 0.1% w/v in the final preparation administered to the patient. |
|
Safety |
The toxicological properties of sodium formaldehyde sulfoxylate have not been fully investigated. However, it is used in the formulation of injection products at a level to 0.1% w/v in the final preparation administered to the patient. Sodium formaldehyde sulfoxylate is moderately toxic by ingestion, and when heated to decomposition it emits toxic fumes of sulfur dioxide and sodium oxide. LD50 (mouse, oral): 4 g/kg LD50 (rat, IP): >2 g/kg LD50 (rat, oral): >2 g/kg |
|
storage |
Store in well-closed, light-resistant containers at controlled room temperature (15–30℃). |
|
Purification Methods |
It crystallises from H2O as the dihydrate and decomposes at higher temperatures. Store it in a closed container in a cool place. It is insoluble in EtOH and Et2O and is a good reducing agent. [X-ray structure: Tuter J Chem Soc 3064 1955.] Note that RONGALITE {HOCH2SO2Na} should not be confused with formaldehyde sodium bisulfite adduct {HOCH2SO3Na} from which it is prepared by reduction with Zn. [Beilstein 1 IV 3052.] |
|
Incompatibilities |
Sodium formaldehyde sulfoxylate is incompatible with strong oxidizing agents; it is decomposed by dilute acid. |
|
Regulatory Status |
Included in the FDA Inactive Ingredients Database (parenteral products up to 0.1% via the IM, IV, and SC routes). Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
InChI:InChI=1/CH3O3S.Na/c2-1-4-5-3;/h2H,1H2;/q+1;-1/rCH3NaO3S/c2-6(4)5-1-3/h3H,1H2
A comparative study of decomposition of ...
α-hydroxysulfinates 1 (table 1) are synt...
-
-
formaldehyd

Sodium; 2,2,2-trifluoro-1-hydroxy-ethanesulfinate

2,2,2-trifluoro-1,1-ethanediol

rongalite
| Conditions | Yield |
|---|---|
|
In water-d2; Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
In water-d2; Yield given. Title compound not separated from byproducts;
|
formaldehyd

rongalite
| Conditions | Yield |
|---|---|
|
With sodium Oleate; sodium hydrogensulfite; zinc; In water; at 94 ℃; for 4h; further added surfactants; influence od electrodeposited zinc on synthesis;
|
86% |
|
With sodium hydrogensulfite; zinc;
|
formaldehyd
water
sodium anilinomethanesulfonate
Aminoiminomethanesulfinic acid
N-sulfomethyl-sulfanilic acid ; methylaniline-p.ω-disulfonate sodium
2-[ethyl(phenyl)amino]acetonitrile
N-phenylglycinonitrile
sodium anilinomethanesulfonate