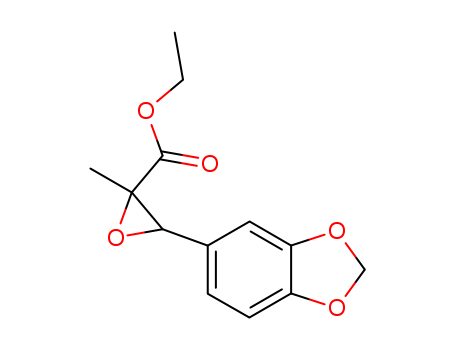
CasNo: 28578-16-7
Molecular Formula: C13H14 O5
|
Synthesis |
To a solution of piperonal (1, 3.00 g, 19.9 mmol) in CH2Cl2?(100 mL) was added (carbethoxyethylidene)triphenylphosphorane (2,14.5 g, 40.0 mmol), and the reaction mixture was stirred for 24 h at room temperature. The mixture was then concentrated at reduced pressure, and the residue was purified by flash column chromatography on silica gel (hexanes/EtOAc,10:1) to afford olefin 3 (4.45 g, 95%) as a colorless oil:?1H NMR (400 MHz, CDCl3) d 7.58 (s, 1H), 6.92 (d, J = 1.7 Hz, 1H), 6.90 (dd, J = 7.9, 1.7 Hz, 1H), 6.82 (d, J = 8.0 Hz, 1H), 5.97 (s, 2H), 4.24 (q, J = 7.1 Hz, 2H), 2.10 (d, J = 1.6 Hz, 3H),1.33 (t, J = 7.1 Hz, 3H);?13C NMR (100 MHz, CDCl3) d 168.7,147.6, 138.3, 129.9, 126.9, 124.6, 109.5, 108.2, 101.2,? 60.7, 14.3,1 4.0; IR (ATR, cm-1): 2981, 2902,1698, 1628, 1502, 1489,1442, 1258, 1224; HRMS (EI) m/z calcd for C13H14O4?(M+) 234.0892, found 234.0895.Fig The synthetic step of PMK ethyl glycidate |
InChI:InChI=1/C13H14O5/c1-3-15-12(14)13(2)11(18-13)8-4-5-9-10(6-8)17-7-16-9/h4-6,11H,3,7H2,1-2H3
piperonal

2-chloro-propanoic acid, ethyl ester

3-benzo[1,3]dioxol-5-yl-2-methyl-oxiranecarboxylic acid ethyl ester
| Conditions | Yield |
|---|---|
|
With sodium ethanolate;
|
piperonal

Ethyl 2-bromopropionate

3-benzo[1,3]dioxol-5-yl-2-methyl-oxiranecarboxylic acid ethyl ester
| Conditions | Yield |
|---|---|
|
With sodium ethanolate;
|
piperonal
2-chloro-propanoic acid, ethyl ester
Ethyl 2-bromopropionate
1-(1,3-benzodioxol-5-yl)-2-propanone